
We used the model E-AIM IV to calculate the aerosol liquid water (ALW) content and pH, and we used the model Visual MinteQ to determine the speciation picture of the most important elements under conditions of both deliquescent aerosol (ALW and pH calculated using E-AIM IV, ambient temperature) and simulated fog. We also determined water-soluble inorganic and organic compounds having ligand properties. For each sample, we determined the solubility and dissolution kinetics of 17 elements in a solution simulating fog water in the winter season in the Po Valley (pH 4.7, T 5 ☌, and water content ∼0.5 g m-3).
In this study, we investigated the impact of fog processing on the solubility and dissolution of metals in PM2.5 samples collected in an urban background site in Padova (Italy). Metals are an important atmospheric aerosol component their impacts on health and the environment depend also on their solubility, dissolution kinetics and chemical form in which they are present in the aerosol (e.g., oxidation state, inorganic salt or oxide/hydroxide, organic complex). Finally, we discuss atmospheric implications and highlight the need for future investigations, particularly with respect to reducing emissions of key acid precursors in a changing world, and the need for advancements in field and laboratory measurements and model tools. Next, we review feedbacks of different acidity regimes on key chemical reaction mechanisms and kinetics, as well as uncertainties and chemical subsystems with incomplete information. Here, we describe the impacts of acidity on the phase partitioning of acidic and basic gases and buffering phenomena. This paper reviews the current state of knowledge on these feedbacks with a focus on aerosol and cloud systems, which involve both inorganic and organic aqueous-phase chemistry. Atmospheric research has made substantial progress in understanding feedbacks between acidity and multiphase chemistry during recent decades. These feedbacks between acidity and chemistry have crucial implications for the tropospheric lifetime of air pollutants, atmospheric composition, deposition to terrestrial and oceanic ecosystems, visibility, climate, and human health. In addition, the acidity of atmospheric aqueous phases, e.g., deliquesced aerosol particles, cloud, and fog droplets, is also dictated by aqueous-phase chemistry. ISBN 0-93.The acidity of aqueous atmospheric solutions is a key parameter driving both the partitioning of semi-volatile acidic and basic trace gases and their aqueous-phase chemistry. Berg “Principles of Bioinorganic Chemistry” University Science Books: Mill Valley, CA 1994. : CS1 maint: uses authors parameter ( link)
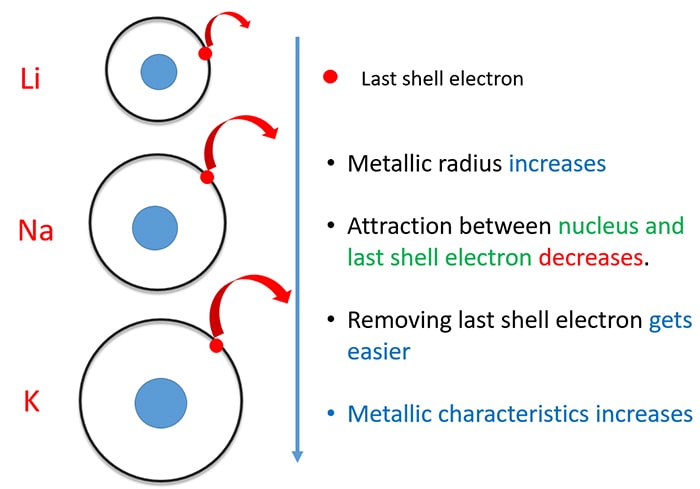
#TRANSITION METALS REACTIVITY SERIES#
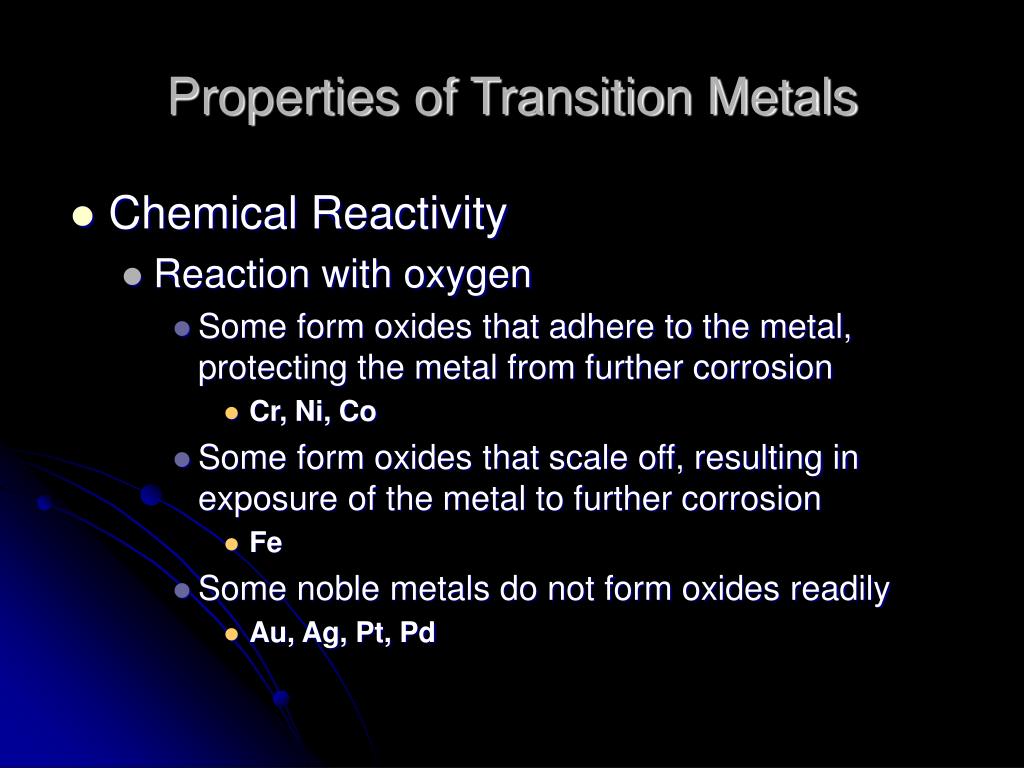
Substitution of Sulfur by Selenium in the Series 2- X = S/Se and Y = S/Se". "Application of a Universal Force Field to Mixed Fe/Mo−S/Se Cubane and Heterocubane Clusters. ^ Axel Kern, Christian Näther, Felix Studt, Felix Tuczek (2004).Bochmann, Manfred (1999), Advanced Inorganic Chemistry (6th ed.), New York: Wiley-Interscience, ISBN 7-5 Albert Wilkinson, Geoffrey Murillo, Carlos A. This property plays a role in the stabilization of Fe(IV) states in the enzyme cytochrome P450. Having filled p-orbitals of suitable symmetry, thiolates are pi-donor ligands. Such acute angles are also seen in the M-S-C angles of metal thiolates. Synthetic analogues can be prepared by combined redox and salt metathesis reactions: 4 FeCl 3 + 6 NaSR + 6 NaSH → Na 2 + 10 NaCl + 4 HCl + H 2S + R 2S 2 Structure and bonding ĭivalent sulfur exhibits bond angles approaching 90°. Thiolate clusters of the type 2− occur in iron–sulfur proteins. This phenomenon is illustrated by the synthesis of cuprous thiolates from cupric precursors:Ĥ HSC 6H 5 + 2 CuO → 2 Cu(SC 6H 5) + (C 6H 5S) 2 + 2 H 2O Consequently, they induce redox reactions with certain transition metals. Thiols and especially thiolate salts are reducing agents. These reactions may proceed by the oxidative addition of the thiol to Fe(0). Some metal centers are oxidized by thiols, the coproduct being hydrogen gas:įe 3(CO) 12 + 2 C 2H 5SH → Fe 2(SC 2H 5) 2(CO) 6 + Fe(CO) 5 + CO + H 2 Organic disulfides oxidize low valence metals, as illustrated by the oxidation of titanocene dicarbonyl: Many thiolate complexes are prepared by redox reactions.
Regarding their mechanism of formation from thiols, metal thiolate complexes can arise via deprotonation of thiol complexes. Thiomersal, a disinfectant, is a metal thiolate complex, being derived from Hg(II) and thiosalicylic acid.


 0 kommentar(er)
0 kommentar(er)
